




Grange Tower Bridge Hotel
P.: + 44 (0) 0207 959 5000
E: towerbridge.groups@grangehotels.com
Please quote CTO169015 when making your booking
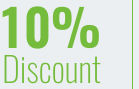
If you are a registered participant and wish to enrol a further new* delegate, please contact Lucia Romagnoli to obtain a 10% discount. This will be applied as soon as the new registration is confirmed.
* This 10% discount is applicable for those who have never attended our event before or who have not attended our event for the past 2 years.
 Welcome to our 2017 Biosimilar Medicines Conference webpage!
Welcome to our 2017 Biosimilar Medicines Conference webpage!
Come and join us for what has become, over the years, the annual gathering of international experts in the field of biosimilar medicines and a flagship event for an ever-growing number of stakeholders.
Following the successful event in 2016 when we celebrated the 10th anniversary of the launch of the first biosimilar medicine in the EU, the Biosimilar Medicines Group will organise its 2017 annual conference ‘Biosimilar Medicines: a game changer for healthcare sustainability’, in London on 23 & 24 March.
Our 15th Biosimilar Medicines Conference will provide insight into the current state of play in the EU and stimulate debate, in a multi-stakeholder setting, on the important role of biosimilar medicines in the sustainability of healthcare systems.
Beyond a comprehensive outlook of key European market access policies, a panel of renowned international regulators will outline the key recent developments in regulatory science and regulatory policy in the EU and other international jurisdictions. Particular emphasis will be placed on strengthening the link between regulators and medical communities as an essential basis for greater understanding and acceptance of biosimilar medicines.

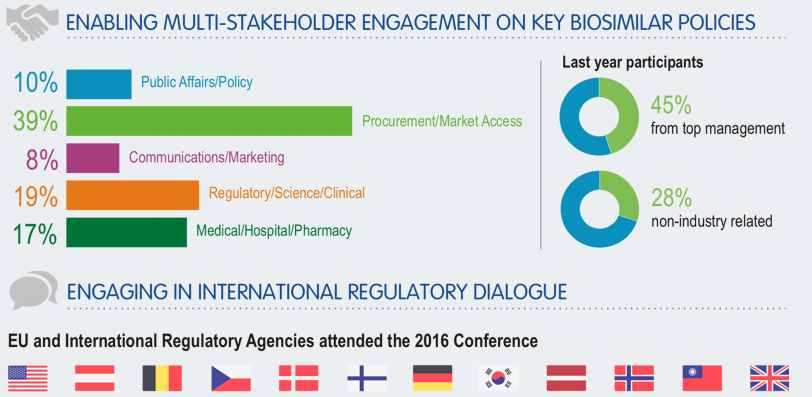
Session B1/L6 – A competitiveness boost: how to strengthen biosimilar medicine developers in Europe?
Session B2 – What are the trends for the use of Biosimilar Medicines in medical practice in Europe?
Session B3 – Improving Access to modern therapies: What can we learn from gainsharing practices?
Session B4 – What is sustainability? – A multi-stakeholder approach to securing the future of healthcare systems
Session B5 – Connecting the Dots? Towards International Regulatory Convergence
Session B6 – Convergence between Regulatory science and Medical practice: closing the loop
Session B7 – What are the Key New International Developments? Any Regulatory Questions? Ask the Regulators!
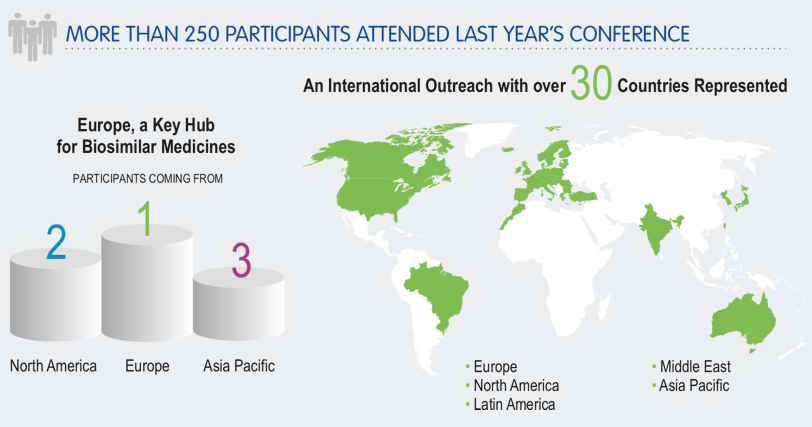

To make the most of this unique opportunity, questions should be formulated generally and be sent by 8 March 2017 to Julie Marechal
Learn more on the legal aspects by also joining our 13th Legal Affairs Conference (more information click here) which will start the day before (22 March) in the same hotel. A 10% discount will be offered to those registering for both events. If you are interested in attending only in part, please send an email to Lucia Romagnoli by the 10 March 2017 with your request and a tailor-made programme fee will be proposed.
Lucia Romagnoli
M: +44 (0) 7 562 87 68 73